Pavilion Publishing and Media Ltd
Blue Sky Offices Shoreham, 25 Cecil Pashley Way, Shoreham-by-Sea, West Sussex, BN43 5FF, UNITED KINGDOM
Learning points
- There is a close relationship between sarcopenia, bone loss, or hip fractures called osteo-sarcopenia.
- Sarcopenia is prevalent between 2% and 37% in community‐dwelling older adults, depending on the sarcopenia definition applied.
- Vitamin D deficiency is associated with sarcopenia, muscle weakness and reduced physical performance.
- Sarcopenia can be identified early through use of fast-screening tools such as the SARC-F questionnaire.
- Inadequate protein intake can also contribute to sarcopenia and decreased function.
Introduction
The older population is increasing worldwide due to advances in medical technology and new treatment options, which has led to an rise in life expectancy.1 Ageing is associated with decline in physical and cognitive function that results in various health issues such as sarcopenia, falls and frailty.2,3
These health issues limit our ability to be independent in normal activities of daily living and cause decline in our quality of life. For example, approximately one‐third of older adults fall at least once a year4 and a median of 4.1% of falls results in fractures.5
Sarcopenia is defined as “a progressive and generalised skeletal muscle disorder involving the accelerated loss of muscle mass and function that is associated with increased adverse outcomes including falls, fractures, disability, and mortality”.6 There is also a close relationship between sarcopenia, bone loss, or hip fractures called osteo-sarcopenia.7
Sarcopenia is prevalent between 2% and 37% in community‐dwelling older adults, depending on the sarcopenia definition applied8 and associated with decreased mobility, impaired standing balance, functional decline, hospitalisation, and mortality.9
Pathophysiology of sarcopenia in old age
There is age-associated decline in the skeletal muscle mass and strength which starts from approximately the fifth decade of life.10 There is decrease in the muscle mass at a rate of 1 to 2% every year after the age of 50.11 The decline is more rapid afterwards in the sixth and seventh decade of life. These morphological changes are reflected in muscle function.
Muscle strength decreases approximately 20–40% in individuals around age 70 compared to young adults of age 20. The loss of muscle strength even increases to 50% in individuals in their nineties.12
From a histological standpoint, the skeletal muscle consists of type I and type II fibers. Type II fast fibers possess a higher glycolytic potential, lower oxidative capacity, and faster response, whereas type I slow fibers are known as fatigue-resistant due to their characteristics such as greater density and content of mitochondria, capillaries, and myoglobin. There is predominant atrophy of type II fibers in sarcopenia along with smaller and fewer mitochondria.13
Development of sarcopenia in old age
The pathological process is multifactorial, and the following changes contribute to development of sarcopenia in old age.
There is increased production of cytokines that have catabolic effects such as IL1, IL6 and TNF alpha14 and the concentration of anabolic cytokines such as IGF-1 is reduced.15
There is a reduction in the level of anabolic hormones such as testosterone, growth hormone, and DHEA and an increase in the level of catabolic hormones e.g. glucocorticoids, myostatin (“myokine” or a hormone-like protein [cytokine] released from muscle, that can act in an autocrine or paracrine fashion).
There is a role of nutritional deficiency and physical inactivity in the development of sarcopenia. Vitamin D deficiency is also widely prevalent in the older population group especially in long-term care institutions such as nursing and residential home facilities.
Vitamin D deficiency is associated with sarcopenia, muscle weakness and reduced physical performance.16 There is emerging evidence that suggests dietary protein supplementation above the recommended dietary amount may be an intervention target to prevent and/or mitigate sarcopenia along with resistance exercise therapy.
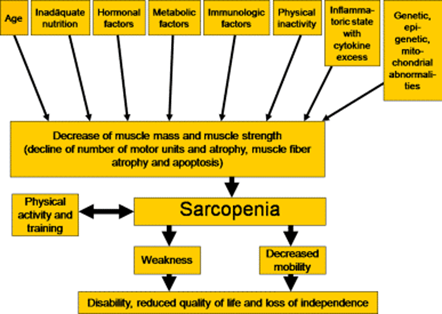
Categories of sarcopenia
Two categories of sarcopenia ‒ primary and secondary ‒ have been recognised in the literature.17
Primary
Sarcopenia is either age-related, or primary, when no other specific cause is evident.
Secondary
It is secondary when causal factors other than ageing are evident. Factors possibly contributing to secondary sarcopenia include physical inactivity owing to limited mobility, disease-related bedrest, or a sedentary lifestyle; poor nutrition owing to undernutrition, inadequate intake of energy or proteins, limited ability to eat, malabsorption or anorexia; and a systemic disease, such as an inflammatory condition (e.g. osteoarthritis, organ failure) or a neurological disorder.
There are two subcategories of sarcopenia:
- Acute: It is termed acute when it lasts for less than six months and is generally associated with an injury or illness.
- Chronic: It is chronic when it lasts longer than six months and usually is related to a progressive condition.
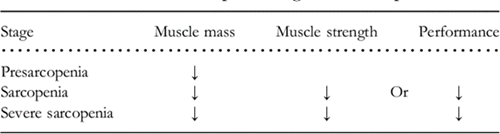
Stages of sarcopenia
The European Working Group on Sarcopenia identifies three stages of sarcopenia:
- Pre-sarcopenia: characterised by low muscle mass and normal muscle strength or physical performance.
- Sarcopenia: characterised by both low muscle mass and low muscle strength or physical performance; and
- Severe sarcopenia: characterised by low muscle mass, low muscle strength, and low physical performance.
Screening for sarcopenia
Sarcopenia can be identified early through use of fast-screening tools such as the SARC-F questionnaire. This is the easiest available measure for screening in the clinic and requires no special equipment. It has been recommended for screening for sarcopenia by the European Working Group on Sarcopenia in Older People.
SARC‐F includes five components: strength, assistance walking, rise from a chair, climb stairs, and falls. SARC‐F items were selected to reflect health status changes associated with the consequences of sarcopenia.18
Scores range from 0-10, with 0-2 for each component.7 Participants with total score higher than 4 are classified as having sarcopenia. Details of SARC-F are shown below.
SARC-F
Strength
How much difficulty do you have in lifting and carrying 10 pounds?
- None=0
- Some=1
- A lot or unable=2
Assistance in walking
How much difficulty do you have in walking across a room?
- None=0
- Some=1
- A lot, use aids or unable=2
Rise from a chair
How much difficulty do you have in transferring from a chair or bed?
- None=0
- Some=1
- A lot, or unable without help=2
Climb stairs
How much difficulty do you have in climbing a flight of 10 stairs?
- None=0
- Some=1
- A lot or unable=2
Falls
How many times have you fallen in the past 1 year?
- None=0
- Some (1-3 falls) =1
- A lot or unable (4 0r more falls) =2
A score equal to or greater than 4 is predictive of sarcopenia and poor outcomes.
Diagnosis of sarcopenia
Diagnosis of sarcopenia is based on documentation of criterion 1 plus criteria 2 or 3
- Criterion 1 Low muscle mass
- Criterion 2 Low muscle strength
- Criterion 3 Low physical performance
Assessment of muscle mass
CT and MRI scan can be used in the diagnosis of sarcopenia to estimate muscle mass however the widespread use is hampered by the high cost and highly qualified personnel needed to do the interpretation. Both modalities are capable of detecting changes in muscle structure.
Although the time necessary for the image acquisition in the case of CT is shorter than for MRI, the radiation exposure is significant.
Dual-energy x-ray absorptiometry (DXA) is a well-established, low-radiation technique used to assess body composition and provides reproducible estimates of appendicular skeletal lean mass.1 Appendicular skeletal muscle mass (ASMM) is a measure of body muscle content, and it correlates with nutrition and physical status.
Bio-electrical impedance analysis (BIA) is a method which estimates the volume of fat and lean body mass based on the relationship between the volume of a conductor and its electrical resistance. The method is not expensive, requires no specialised staff and is relatively easy to use in clinical practice, both on ambulatory subjects or on hospitalised patients. Reference values have been established for older individuals.20
Assessment of muscle strength
The Jamar dynamometer, or similar hydraulic dynamometer, is the gold standard for this measurement. However, for patients with advanced arthritis, the design of this dynamometer may be a limitation. A pneumatic dynamometer, such as the Martin vigorimeter, may be a good alternative. With this device, patients try to squeeze rubber balls (available in three sizes) with the same protocol as that used for the Jamar dynamometer. A variety of thresholds of grip strength have been proposed to characterize low muscle strength, ranging from 16 to 20kg for women and 26–30kg for men.
Assessment of performance
The second part of assessment depends on physical performance that will use two tests:
First is the Short Physical Performance Battery (SPPB) that evaluates balance, gait, strength and endurance by examining an individual’s ability to stand with the feet together in side-by-side, semi-tandem and tandem positions, time to walk 8ft and time to rise from a chair and return to the seated position five times.
The second test is Timed get-up-and-go test (TUG) which measures the time needed to complete a series of functionally important tasks. TUG requires the subject to stand up from a chair, walk a short distance, turn around, return and sit down again.
Treatment of sarcopenia
Diet
Inadequate protein intake can also contribute to sarcopenia and decreased function. A prospective cohort study found that adults aged 70 to 79 with protein intake ≤0.8 g/kg/day (the recommended dietary allowance were at greater risk of developing mobility limitations over six years of follow-up than those with protein intake ≥1.0 g/kg/day.21
Screening tools for malnutrition are intended for the quick identification of patients at risk of malnutrition, for more in-depth nutritional assessment, or for identifying patients at risk of developing sarcopenia or even increased risk of mortality. The combination of screening and assessing for malnutrition and sarcopenia is recommended to screen for the presence of malnutrition-sarcopenia syndrome in at-risk patient populations, particularly older adults in clinical settings. The common tools used are Mini Nutritional assessment-Short Form (MNA-SF) and Simplified Nutritional Appetite Questionnaire –SNAQ.
Vitamin D
Vitamin D is associated with neuromuscular performance but results on the association of vitamin D levels with muscle strength and physical performance in the older persons are somehow discordant. Some studies show a significant association between low vitamin D levels and poor physical performance, assessed by handgrip strength and the short physical performance battery – a lower-extremity objective performance based evaluation tool, including raising five times from a chair, maintaining balance in three more challenging positions and the four-meter usual walking speed test.22
A randomised study on sedentary older men with a vitamin D 23
Additional studies are needed to clarify the effect of vitamin D supplementation on skeletal muscle and its optimal serum levels to maintain a good physical function in advanced age.
Progressive Resistance Training (PRT)
Low physical activity and sedentary lifestyle are main causes of sarcopenia, exercise is a primary strategy in the prevention and treatment of sarcopenia. Both aerobic training and resistance training can improve the rate of decline in muscle mass and strength with age.24
Aerobic exercise has beneficial impact on sarcopenia by improving skeletal muscle insulin sensitivity; stimulating skeletal muscle hypertrophy; and increasing skeletal muscle mass. Examples include treadmill, cycling, jogging, light swim etc.
In comparison to aerobic training, resistance training has a greater effect on increasing muscle mass and strength and attenuates the development of sarcopenia.24
Resistance training is a form of exercise in which muscle contracts against an external load. Equipment commonly used to perform resistance training includes free weights, exercise machines, body weight, and elastic bands.25 Examples include bicep curl, arm raise, tennis ball squeeze and lawn mower pull exercises for upper extremity strengthening and standing leg curl, hip extension, hip flexion, knee extension, side hip raise, and toe stand exercises for lower extremity strengthening performed at least one set of 8–12 repetitions.
Testosterone and sarcopenia
Hypogonadism is one of the causes of sarcopenia. Testosterone is a steroid hormone produced by the Leydig cells of the testes.26 After the age of 30 years, testosterone levels decrease at a rate of 1% per year.27 Therefore up to 70% of men older than the age of 70 years, are likely to have reduced testosterone levels.28
Studies have shown a relationship between muscle mass and its function with testosterone.26 In a study by Baumgartner RN, et al. (1999), elderly men’s muscle mass was associated with serum free testosterone and insulin like growth factor 1 (IGF1).29 Another study shows that age related reduced testosterone levels is associated with reduced capacity of maximum voluntary neuromuscular performance in older people.30
Studies have shown that in humans, testosterone treatment increased type I and type II muscle fibers.31,32 This could be a potential drug treatment for sarcopenia, however its side effects of increased hematocrit levels and increased risk of cardiovascular disease is a concern.26
Other treatment options in development
There are many medications in phase 1 or 2 trials for treatment of sarcopenia. These include activin receptor antagonist, myostatin or activin inhibitor, and selective androgen receptor modulator (SARM).
Dr Irfan Muneeb, Consultant Geriatrician
Dr Sakhawat Ali, Specialty Doctor, Medicine
Dr. Muhammad Josheel Naveed, Consultant Physician
References
- Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013 (London, England), 381(9868), 752–762. https://doi.org/10.1016/S0140-6736(12)62167-9
- Maresova P, Javanmardi E, Barakovic S, et al. (2019). Consequences of chronic diseases and other limitations associated with old age – a scoping review. BMC public health, 19(1), 1431. https://doi.org/10.1186/s12889-019-7762-5
- Shafiee G, Keshtkar A, Soltani A, et al. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord 2017;16:21.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged >/=65 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65:993–998
- Morrison A, Fan T, Sen SS, Weisenfluh L. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res 2013;5:9–18.
- Cruz-Jentoft, Alfonso J, et al. “Sarcopenia: revised European consensus on definition and diagnosis.” Age and ageing vol. 48,1 (2019): 16-31. doi:10.1093/ageing/afy169
- Huo YR, Suriyaarachchi P, Gomez F, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc. 2015 Apr;16(4):290-5. doi: 10.1016/j.jamda.2014.10.018. Epub 2014 Dec 12. PMID: 25512216.
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014 Nov;43(6):748-59. doi: 10.1093/ageing/afu115. Epub 2014 Sep 21. PMID: 25241753; PMCID: PMC4204661
- Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000 Jul;89(1):81-8. doi: 10.1152/jappl.2000.89.1.81. Erratum in: J Appl Physiol (1985). 2014 May 15;116(10):1342. PMID: 10904038.
- Sehl ME, Yates FE. Kinetics of human aging: I. Rates of senescence between ages 30 and 70 years in healthy people. J Gerontol A Biol Sci Med Sci. 2001;56:198–208
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle, strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci, 61(10): 1059–1064, 2006.
- Ogawa, S., Yakabe, M. & Akishita, M. Age-related sarcopenia and its pathophysiological bases. Inflamm Regener 36, 17 (2016). https://doi.org/10.1186/s41232-016-0022-5
- Roubenoff R, Harris TB, Abad LW, et al. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci, 53: M20–M26, 1998.
- Harris TB, Kiel D, Roubenoff R, et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc, 45: 133–139, 1997
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861.
- Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177-80
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256
- Levine JA, et al. Measuring leg muscle and fat mass in humans: comparison of CT and dual-energy X-ray absorptiometry. J Appl Physiol. 2000;88:452–6
- Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23
- Houston DK, Tooze JA, Garcia K, et al; Health ABC Study. Protein Intake and Mobility Limitation in Community-Dwelling Older Adults: the Health ABC Study. J Am Geriatr Soc. 2017 Aug;65(8):1705-1711. doi: 10.1111/jgs.14856. Epub 2017 Mar 17. PMID: 28306154; PMCID: PMC5555791
- Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: The InCHIANTI study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440.
- Levis S., Gòmez-Marìn O. Vitamin D and Physical Function in Sedentary Older Men. JAGS. 2016;65:323–331. doi: 10.1111/jgs.14510
- Burton LA, Sumukadas D. Optimal management of sarcopenia. Clin Interv Aging. 2010;7:217–28
- Mangione KK, Miller AH, Naughton IV. Review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90:1711–5. doi: 10.2522/ptj.20100270
- Shin MJ, Jeon YK, Kim IJ. Testosterone and Sarcopenia. Review Article pISSN: 2287-4208 / eISSN: 2287-4690 World J Mens Health 2018 September 36(3): 192-198 https://doi.org/10.5534/wjmh.180001
- Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997;46: 410-3
- Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Int J Androl 2009; 32:1-10
- Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 1999;107: 123-36.
- Häkkinen K, Pakarinen A. Muscle strength and serum testosterone, cortisol and SHBG concentrations in middle-aged and elderly men and women. Acta Physiol Scand 1993;148: 199-207
- Kadi F. Adaptation of human skeletal muscle to training and anabolic steroids. Acta Physiol Scand Suppl 2000;646: 1-52
- Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc 1999;31: 1528-34


