Pavilion Publishing and Media Ltd
Blue Sky Offices Shoreham, 25 Cecil Pashley Way, Shoreham-by-Sea, West Sussex, BN43 5FF, UNITED KINGDOM
Hormone therapy in prostate cancer
This review summarises important androgen deprivation therapy in management of prostate cancer, looking at new as well as established treatment options, and associated medical complications.
Prostate cancer is the commonest cancer in men in the UK, with an incidence of 40,975 men diagnosed and 10,721 deaths in 2010. One in nine men have the diagnosis of prostate cancer; with the largest number of cases being diagnosed in the 70-74 years age group. Although there has been a rise in prostate cancer incidence, this has not been reflected by its mortality rate; with five year survival rates remaining at around 70% to 80%.1
Androgen deprivation therapy
Most prostate cancer cells are driven by testosterone, and hence treatment aimed at lowering serum testosterone, known as androgen deprivation therapy plays an important role in prostate cancer management. In 1941, Charles B. Huggins demonstrated this Nobel winning discovery in his seminal paper “Studies on prostate cancer. I. The effect of castration, of oestrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate”. Surgical castration (orchidectomy) was found to slow overall progression of disease and control painful symptoms from prostate cancer. Although it remains gold standard for first-line treatment of metastatic prostate cancer, surgical removal of testicles has a strong psychological impact on men.
Nowadays, medical castration has largely replaced the need for orchidectomy, with a castrate level of testosterone achieved within weeks. Androgen deprivation therapy remains first line management in metastatic prostate cancer and is also used in the neoadjuvant and adjuvant setting of locally advanced or high risk localised disease.
Luteinising hormone releasing hormone agonist
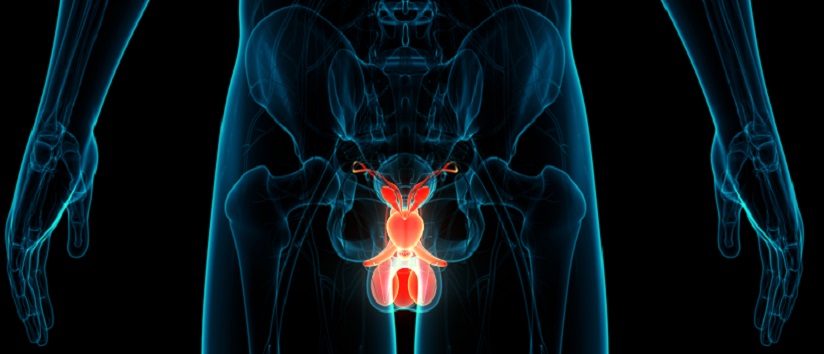
The most established form of hormonal agent used in prostate cancer is luteinising hormone-releasing hormone agonists (LHRHa), which can be delivered as monthly, three monthly or six monthly subcutaneous injections. LHRH is produced by the hypothalamus, causing the release of LH from the anterior pituitary, which in turn acts on Leydig cells of the testes to produce testosterone (Figure 1). Testosterone surge causes a negative feedback action on the anterior pituitary, which then reduces production of testosterone to a castrate level.
Treatment with LHRH agonist can cause an initial testosterone surge and theoretical tumour flare, which is prevented by administration of an oral anti-androgen two weeks before and two weeks after the first LHRH agonist injection. Goserelin (Zoladex) is an example of an LHRH agonist, which came into practice in 1987, and has been in use since. Although medical castration with LHRH agonist takes up to four weeks to achieve castrate level of testosterone compared to 12 hours in surgical castration, they have been proven to be equally as effective.2
Gonadotrophin releasing hormone (GnRH) antagonist
Another rational way of hormonal manipulation is by inhibiting GnRH.3 One example of a GnRH antagonist is Degarelix, which competitively binds to pituitary GnRH receptors causing a rapid decline in gonadotrophins; luteinising hormone (LH) and follicle stimulating hormone (FSH). This will in turn reduce secretion of testosterone by the testes. The advantage of GnRH antagonist therapy includes its rapidity of effect and the avoidance of potential tumour flare. A phase III randomised trial of 610 patients showed a decrease in serum testosterone to below 0.5ng/ml in 96% of patients within three days of starting Degarelix treatment.3 It is delivered via monthly subcutaneous injections.
Anti-androgen
In contrast to drugs manipulating hormonal pathway upstream, anti-androgen acts downstream by binding to cancer cell surface receptors. Agents such as bicalutamide or flutamide bind to cancer cell surface receptors preventing testosterone from stimulating growth. As a result, it causes a rise in serum testosterone level and has a different side effect profile compared to LHRH agonist. Anti-androgens are delivered conveniently as oral tablets in daily preparations.
Combined androgen blockade (CAB)
After a period of time, upstream inhibition of testosterone by LHRH agonist will be less efficacious due to drug resistance. One way of overcoming this is by combining upstream blockade of androgen production together with downstream targets against androgen receptors, a strategy well known as “Combined Androgen Blockade”. This can be used as initial combination therapy but in the UK it is more common to use “step up” CAB with the addition of an anti-androgen (eg. bicalutamide 50mg daily) at the time of first resistance to LHRH agonist therapy.
After a period of time, the same pattern repeats itself and disease progression continues despite CAB. Anti-androgen can cause a paradoxical agonistic effect on androgen receptors of cancer cells, hence driving PSA rise. A strategy developed to combat this paradoxical effect is by simply stopping the anti-androgen but continuing on LHRH agonist therapy.
Castrate resistant prostate cancer (CRPC)
Prostate cancers that are progressing despite castrate testosterone levels are considered castration resistant.4 The terminology of castrate resistant prostate cancer (CRPC) should not be confused with hormone resistant prostate cancer (HRPC), which indicates resistance to all hormonal measures. Cancer cells in CRPC still respond to hormonal measures and may indeed be supersensitive to very low levels of androgen.
As such, continued androgen deprivation with an LHRH agonist and the addition of further hormonal manipulation with anti-androgen withdrawal, oestrogens or corticosteroids, may all be effective treatment strategies in metastatic castrate resistant prostate cancer (mCRPC).4 Diethylstilboestrol is usually prescribed as oral preparation with combination of aspirin or warfarin to reduce risks of thrombotic complications.
Exciting developments in prostate cancer therapies have produced newer hormonal agents to treat mCRPC and these include enzalutamide and abiraterone.
Enzalutamide
Enzalutamide was developed with the intention to improve androgen receptor (AR) affinity compared to other anti-androgens (bicalutamide and flutamide), and avoid its partial agonistic activity.5 Theoretically, enzalutamide achieves this by inhibiting AR nuclear translocation, inhibiting association of the AR with DNA and competitive androgen binding to the AR.6 Enzalutamide is currently being licensed by the FDA in the post chemotherapy setting and is awaiting a NICE review.
Abiraterone
Extensive study was done to understand mCRPC, and its mechanisms of resistance. Increased AR expression, mutation in the AR gene and activation of the AR due to cross talk via other signalling pathways are some of the clever methods adopted by cancer cells to overcome drug targets.7,8 Despite resistance, there seems to be continued dependence on AR, coupled with increasing the level of testosterone to a pre-castration level. This suggests the possibility of de novo synthesis of testosterone from pregnenolone and other precursors within the tumour.9 The discovery of high expression of steroid biosynthesis enzyme, CYP17, is seen in prostate cancer cells, consolidating this theory further.8 An elegant method to target this pathway was developed with the drug abiraterone, a potent, selective and irreversible inhibitor of the CYP17 enzyme. To prevent increase in mineralocorticoids or change in fluid imbalance, abiraterone is delivered together with prednisolone. It is prescribed at a dose of 1000mg daily, in a convenient oral preparation.
Evidence supporting hormone manipulation
Androgen deprivation therapy is the first-line management in patients with advanced and metastatic prostate cancer. It gives a response rate of 85% for an average of three years.10 Timing of therapeutic initiation remains a topic of discussion, given most men lack symptoms on presentation.
To gain insight into this dilemma, a randomised controlled trial by the Medical Research Council11 compared immediate with deferred hormonal therapy in advanced prostate cancer. The trial involving 943 men found immediate treatment to be advantageous as it showed reduction in distant progression (26% in the immediate group compared to 45% in the deferred group). Furthermore, pathological fractures, spinal cord compression and ureteric obstruction were twice as common in deferred patients. This evidence is in support of early treatment with a hormonal agent, which will reduce complications of advanced prostate cancer.
In locally advanced prostate cancer, adjuvant treatment with hormonal therapy after radical external beam radiotherapy (EBRT) is advocated. The EORTC 22,863 trial12 found an increased 10 year survival in patients treated with EBRT and three years of adjuvant goserelin compared to patients treated with EBRT alone (58.1 versus 39.8%, HR = 0.60, CI: 0.45-0.80, p = 0.0004).
The phase III EORTC trial and the RTOG 92-02 also showed overall survival benefit as well as a reduction in distant progression free survival with goserelin.13
Investigating a different agent, the International Early Prostate Cancer14 study evaluated the efficacy and tolerability of adding an anti-androgen (bicalutamide 150mg daily) to standard care (prostatectomy, radiotherapy or watchful waiting) in 8,113 patients. A significant improvement in objective progression free survival and overall survival was demonstrated in favour of bicalutamide for locally advanced patients when given as adjuvant to radical radiotherapy (HR=0.70 (CI 0.51 to 0.97), p=0.03).
As anti-androgen treatment causes less erectile dysfunction and does not reduce bone mineral density, it is an alternative agent in this sub-group of patients but at the expense of an increased risk of gynaecomastia and mastalgia.
Combining LHRHa and anti-androgen is a popular second-line treatment in patients who progress with LHRHa. In 2000, the Prostate Cancer Trialists’ Collaborative Group published a meta-analysis of 27 trials comparing Combined Androgen Blockade (CAB) with monotherapy.15
It found CAB to be modestly beneficial with five year survival of 25.4%, compared to monotherapy at 23.6%. However, it transpired the choice of anti-androgen used for CAB has an impact on outcome. CAB with the steroidal anti-androgen, cyproterone acetate is associated with an increased risk of death (reduction in five year survival of 2.8%). Conversely, CAB with non-steroidal anti-androgens, such as flutamide confers an improvement of 2.9% in five year survival over monotherapy.
In metastatic castrate resistant prostate cancer (mCRPC), two randomised, double-blind phase III trials have shown efficacy of abiraterone in post-chemotherapy as well as chemotherapy naïve patients. Abiraterone was initially tested in patients who have progressed on docetaxel chemotherapy. In the COU-AA 301 trial, patients were randomised between abiraterone with prednisolone against placebo and prednisolone.15 Significant improvement in overall survival with abiraterone plus prednisolone was seen, with prolongation of life expectancy of about four months (15.8 months versus 11.2 months, respectively; HR 0.74; 95% CI 0.64-0.86; p< 0.001).15 Abiraterone has been approved by NICE in this setting.
In the pre-chemotherapy setting, a second trial (COU-AA-302) was carried out comparing abiraterone plus prednisolone against placebo in asymptomatic or mildly symptomatic patients with metastatic castrate resistant prostate cancer. This study found improvement in radiographic progression free survival of 16.5 versus 8.3 months (HR 0.53; 95% CI 0.45-0.62; p<0.001).16 However, although a strong trend towards improved overall survival was seen, it did not reach the pre-specified boundary for significance p‰¤0.001.16
Based on the trial, the European Medicines Agency has approved abiraterone for asymptomatic mCRPC patients, in whom chemotherapy is not yet clinically indicated and a NICE appraisal is awaited.
Enzalutamide was investigated in a phase III randomised controlled trial of mCRPC patients who progressed despite chemotherapy. The AFFIRM study6 showed prolongation of median OS with enzalutamide by nearly five months compared to placebo (18.4 months versus 13.6 months respectively (HR 0.631; 95% CI 0.53-0.75; p<0.0001).
Enzalutamide is also found to be superior to placebo in all secondary endpoints, including PSA, soft tissue and quality of life response rate, as well as time to first skeletal related event. FDA has approved enzalutamide to be used in patients who have had docetaxel chemotherapy and NICE appraisal is awaited.
There are many other new and exciting compounds in clinical trials offering further options for these men both in terms of increased survival and improved quality of life. These new hormone therapies are generally well tolerated.
Therapeutic strategies in prostate cancer
With the advent of newer therapeutic agents, clinicians need to think carefully and strategise in order to maximise each therapy for the benefit of their patient. The rapidity of drug development leaves a gap in the evidence behind best drug sequencing strategy. Key aspects that need to be considered include patient, disease and therapeutic factors summarised.
Traditionally, patients who develop androgen resistance (mCRPC) would be considered for chemotherapy, mainly with docetaxel cytotoxic agent.17 Chemotherapy is perhaps best for patients with, heavy tumour burden or visceral metastatic lesions, which can benefit from rapid response to therapy. This depends heavily upon their fitness status, as docetaxel is known to cause side effects such as bone marrow suppression, lethargy, peripheral neuropathy, hypersensitivity reaction and myalgia.
In chemotherapy naïve patients, first-line treatment with LHRH agonists, followed by addition of anti-androgens on progression of disease (combined androgen blockade) is common practice in the UK. Third line treatment choices in mCRPC when chemotherapy is not yet indicated are abiraterone, diethylstilboestrol and dexamethasone. Enzalutamide is currently being investigated in the pre-chemotherapy setting.
In the post chemotherapy setting, patients with progressive mCRPC can be treated with abiraterone and we await the NICE review for enzalutamide. However, enzalutamide offers the potential advantage of steroid free administration.
As already clear, an adaptive approach is desirable in making therapeutic decisions for patients.
Hormonal manipulation
Hormonal manipulation has well documented toxicities, which are worth noting, especially as most patients with prostate cancer die with the disease rather than from it. Toxicities mainly due to reduction of serum testosterone are erectile dysfunction, loss of libido, hot flushes, osteoporosis, reduced lean muscle mass and strength, breast swelling, mastalgia, weight gain, tiredness, anaemia, mood swings, depression and metabolic complications.
Androgen deprivation therapy is thought to increase risk of cardiovascular disease due to interference with the cardioprotective effect of testosterone, causing central obesity, lipid alterations and insulin resistance.18,19 In his observational study of 14,597 men, Keating et al19 showed treatment with LHRH agonists not only increased risks of coronary heart disease (adjusted Hazard Ratio (aHR) 1.19), MI (aHR1.28), sudden cardiac death (aHR 1.35), but also stroke (aHR 1.22) and diabetes (aHR 1.28). Clinicians must aim to reduce these risks by regular monitoring of weight, waist circumference, BMI, lipid profile and fasting blood glucose with early referral for treatment when abnormalities are detected.
Loss of bone mineral density is a recognised risk factor of androgen deprivation therapy (ADT), which can lead to development of fractures. Retrospective analysis of over 50,000 men found 19.4% of those treated with ADT had fractures compared to 12.6% without ADT (p<0.001).20 Real risk of osteoporosis means patients must be empowered with pertinent information on diet and exercise, which can help reduce their risk of fracture in the long run.
Abiraterone has an unique side effect profile, explained by its mode of action. Owing to its effects on glucocorticoid pathways, abiraterone is associated with toxicities related to mineralocorticoid excess, including fluid retention and oedema, hypokalaemia and hypertension, as well as cardiac disorders and abnormalities of liver function tests. As it is dosed continuously, careful and frequent monitoring of blood pressure and blood tests are imperative to manage its side effects.
Enzalutamide’s toxicities are similar to anti-adrogens such as lethargy, hot flushes and diarrhoea. In its phase III trial, a small percentage of patients with neurological risk factors (0.6%) were noted to have seizures upon starting enzalutamide.
The described toxicities should not prevent the use of hormonal therapy, but early prevention and detection are paramount.
Summary
After 70 years of investigating hormone therapy for prostate cancer, the role of testosterone and androgen receptor remains at the epicentre of therapeutic intervention. Future research and drug development is still concentrated around refining the targets to combat drug resistance. Drug sequencing in a changing landscape is an imperative issue that requires careful consideration. Ultimately, an adaptive approach balancing drug efficacy and toxicities, disease and patient factors is key in managing prostate cancer successfully.
Conflict of interest: none declared
References
1. Cancer Research UK. Prostate cancer statistics. Available at: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/prostate/ Accessed 12/08/13
2. Kaisary AV, Tyrrell CJ, Peeling WB, Griffiths K. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 1991; 67(5): 502-508.
3. Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008; 102(11): 1531-38
4. Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2008; 53(1): 68-80
5. Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009; 324(5928): 787-90.
6. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367(13): 1187-97
7. Attar RM, Takimoto CH, Gottardis MM. Castration-resistant prostate cancer: locking up the molecular escape routes. Clin Cancer Res 2009; 15(10): 3251-55.
8. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008; 68(11):4447-4454
9. Mohler JL, Gregory CW, Ford OH 3rd, et al. The androgen axis in recurrent prostate cancer. Clin.Cancer Res 2004; 10(2): 440-48
10. Mcleod DG. Hormonal therapy: historical perspective to future directions. Urology 2003; 61(2 Suppl 1): 3-7
11. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. Br J Urol 1997; 79(2): 235-46
12 Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. The Lancet Oncology 2010; 11(11): 1066-73
13. Horwitz EM, Bae K, Hanks GE, et al. Ten-Year Follow-Up of Radiation Therapy Oncology Group Protocol 92-02: A Phase III Trial of the Duration of Elective Androgen Deprivation in Locally Advanced Prostate Cancer. Journal of Clinical Oncology. 2008; 26(15): 2497-504.
14. Iversen P, McLeod DG, See WA, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer.. BJU International 2010; 105(8): 1074-81
15. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. The Lancet Oncology 2012; 13(10): 983-92
16. Ryan CJ, Smith MR, De Bono JS, et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N Engl J Med 2013; 368(2): 138-148
17. Tannock IF, De Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351(15): 1502-12
18. Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J. Clin. Oncol. 2006; 24(24): 3979-83.
19. Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy: Observational Study of Veterans With Prostate Cancer. JNCI Journal of the National Cancer Institute 2009; 102(1): 39-46
20. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of Fracture after Androgen Deprivation for Prostate Cancer. N Engl JMed 2005; 352(2): 154-64.