Pavilion Publishing and Media Ltd
Blue Sky Offices Shoreham, 25 Cecil Pashley Way, Shoreham-by-Sea, West Sussex, BN43 5FF, UNITED KINGDOM
First published January 2007; Updated April 2021
Varicella is a highly contagious childhood disease, caused by infection with the varicella zoster virus (VZV). It causes two separate diseases: varicella, commonly referred to as chickenpox, and herpes zoster or shingles. Once a person recovers from varicella, the VZV virus persists in latent form in the sensory nerve ganglia.
The latent virus can be reactivated and emerges as shingles. In extreme cases internal organs €” such as lung, liver or brain €” can be affected.1 The annual incidence is estimated at 3/1000. The incidence increases with age reaching 11/1000 in those over 80 years. Approximately, 50% of individuals reaching 90 years of age will have had shingles.2
- Further reading: Shingles: why is it on the rise?
A second attack may occur in approximately six to 14%.3 In the UK, approximately 170,000 people develop shingles a year.4 A recent US study shows that from 1998€“2003 there was an increase of 90% in age standardised rates of shingles infection.5 The effects of an acute attack on older people is often very profound and in more frail older people may lead to hospital admission.
Depending on the applied definition, between nine to 34% of patients develop postherpetic neuralgia (PHN)6 and the main risk factor is increasing age. Although a variety of definitions of PHN have been used, the pain associated with shingles has three phases: an acute herpetic neuralgia that accompanies the rash and lasts for about 30 days after onset, a subacute herpetic neuralgia that lasts for 30€“120 days and PHN defined as pain that persists for greater than 120 days.
Epidemiology
Shingles is caused by a localised infection with VZV and represents a reactivation of the virus that has lain dormant in the sensory ganglia since a primary infection with chickenpox. The inflammation of the peripheral nerve and skin damage are supposedly responsible for the pain.7 The factors that precipitate reactivation of VZV are unknown but immunosuppression such as seen in older age, immunocompromised patients and HIV infection increase risk.
With shingles there are usually complaints of pain prior to the appearance of any rash, The pain is in the area of skin (dermatome) innervated by the infected nerve. It is described as burning, aching, electric shock-like or unbearable itching, and is associated with dysaesthesias, paraesthesias, hyperalgesia and allodynia (production of pain by innocuous stimuli). Patients may present at this time to medical practitioners but the diagnosis may not be apparent due to the lack of any cutaneous manifestations.
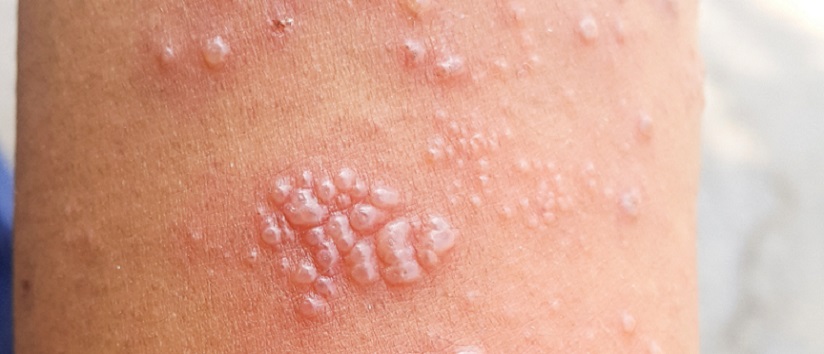
It is important to suspect shingles when the pain seems to be over a distinct dermatome and it is useful to advise the patient to return as soon as possible if the typical rash appears €” typically fluid-filled blisters. It is usually present for seven days but can last for two to three weeks.
The term zoster-associated pain may also be used to describe the pain continuum from the acute event until development of PHN (three months). The incidence of PHN at three months ranges from 17€“60%8 with 48€“56% of those continuing to suffer at 12 months.9,10
Immunity to VZV plays a major part in the pathogenesis of shingles. Cell mediated immunity to VZV is a major determinant of the risk and severity. While levels of antibody to VZV remain relatively constant with increasing age, the increased incidence of shingles (and PHN) in older adults is closely linked to a progressive age-related decline in cell mediated immunity to VZV.
An episode of shingles boosts immunity to VZV, effectively immunising against a further episode, and recurrences are rare among immunocompetent people for this reason. Management Intervention for shingles as early as possible is recommended in order to accelerate healing, limit the severity of acute and chronic pain, and reduce complications. Antiviral use is recommended for people who present within 72 hours of onset of the rash unless there are specific risk factors or increased likelihood of complications. Ophthalmic opinion is advised for those with ophthalmic involvement.
In a recent UK study only 54% received antiviral therapy within 72 hours; a further 25% received treatment outside this timeframe, mainly due to late presentation.11
Complications of shingles
The complications of shingles range from those related to the rash €” eg, superadded infection €” to complications related to particular dermatomes affected (ophthalmic, facial) and the major complication of PHN. Problems with systemic involvement are rare. Herpes zoster ophthalmicus occurs when VZV is reactivated in the ophthalmic division of the trigeminal nerve. Herpes zoster ophthalmicus represents up to one-fourth of all cases of herpes zoster.
Most patients with herpes zoster ophthalmicus present with a periorbital vesicular rash distributed according to the affected dermatome. A minority may also develop conjunctivitis, keratitis, uveitis and ocular cranial nerve palsies. Permanent sequelae of ophthalmic zoster infection may include chronic ocular inflammation, loss of vision and debilitating pain.12
The strict definition of the Ramsay Hunt syndrome is peripheral facial nerve palsy accompanied by an erythematous vesicular rash on the ear (zoster oticus) or in the mouth.
J Ramsay Hunt, who described various clinical presentations of facial paralysis and rash, also recognised other frequent symptoms and signs such as tinnitus, hearing loss, nausea, vomiting, vertigo and nystagmus. He explained these eighth nerve features by the close proximity of the geniculate ganglion to the vestibulocochlear nerve within the bony facial canal. It is now known that varicella zoster virus (VZV) causes Ramsay Hunt syndrome.
Compared with Bell’s palsy (facial paralysis without rash), patients with Ramsay Hunt syndrome often have more severe paralysis at onset and are less likely to recover completely. In the only prospective study of patients with Ramsay Hunt syndrome, 14% developed vesicles after the onset of facial weakness.
Thus, Ramsay Hunt syndrome may initially be indistinguishable from Bell’s palsy. PHN is the most common complication of shingles, usually defined as pain that persists for greater than 120 days after the onset of rash. In a recent UK study, 27% of patients experienced PHN at three months.11
Approximately 50% of these people will have pain one year after the rash appears and at this stage further resolution is limited.3 PHN in older people A major, frequently cited study on shingles and PHN showed that PHN increased with age, from 7.4% in 50€“59 year age group to 34.4% in those over 80 years.13
From this study using the definition of pain persisting more than thee months after the rash, 8.8 per cent of people had PHN. The incidence in the Shingles Prevention Study (SPS)14 was 11.12 cases per 1000 patient years in people over 60 years.
The incidence rate in the placebo group in SPS was three times higher in those over 70 years compared to the 60€“69 year old group. There are a number of predictors for those at most risk of PHN:15 advanced age; significant or prolonged prodromal pain; severity of pain at onset of rash; severe skin rash at presentation; female gender; and ophthalmic location. The age risk seems to be relevant in even healthy individuals over 50 years old.16
It is not clear why the prodromal/acute pain severity or rash severity may lead to more pain, but this may be related to more extensive VZV reactivation which may cause more tissue damage during the acute phase of shingles. In the study by Coen et al15 only age over 50 and Visual Analogue Scale >five were predictive of pain at three months while ophthalmic involvement was predictive at six months.
In older people PHN can have profound effects on mood, the ability to perform activities of daily living and overall quality of life. Depression and anxiety are common and there may be problems with general functional ability resulting in further depression and social isolation. Sleep disruption is also common and all these effects compound so that the individual may be severely disadvantaged. The problem with PHN is related to the attempts to manage the pain in an older population.
The early use of antiviral therapy appears to accelerate the resolution of PHN, but it is not clear whether use of these agents reduces the number of patients developing PHN. Because the pain is neuropathic in origin the attempts to relieve focus on the use of conventional and adjunctive analgesic therapy.
This approach often takes place first in primary care, but the nature and persistence of PHN can lead to multiple consultations and referral to pain services. Indeed, 40€“50 per cent patients with PHN do not respond to any treatment.17 Treatment recommendations for PHN as recommended by the American Academy of Neurology.18
The effective options include tricyclic antidepressants, gabapentin and pregabalin, opioids and topical lidocaine. For all of these medications there may be issues around tolerability and safety in older people, depending on the individual. These need to be balanced against efficacy in such a problematic condition. Tricyclic antidepressants are often associated with confusion and peripheral anticholinergic symptoms as well as cardiac concerns. There is little to choose between preparations. Gabapentin can be associated with dizziness and somnolence in older people and the dosage may need to be reduced, bearing in mind there is often reduced renal function present.
Adverse events may also be a feature of pregabalin. Long-acting oral opioid preparations and tramadol also provide relief in PHN. Again there is little evidence to recommend choice of a particular preparation. The issues in older people are about achievement of the correct dosing along with avoidance of opioid toxicity, and with tramadol the issue is often that the drug is either well tolerated and effective or that there are immediate problems with intolerance.
There are insufficient data to recommend one opioid over another. Lidocaine gel (5%) has been shown to be effective and a lidocaine patch has been licensed in the US for treatment of PHN. The lidocaine patch seems to be very well tolerated, with little systemic absorption. This preparation could soon be available in the UK.19 PHN is difficult to treat in an older population. The interventions may involve several trials of the effective medications discussed above. Adverse events with these therapeutic options are common in older people. These factors lead to repeated, sometimes frequent, consultations and referrals to pain services.
Care of the elderly view
Shingles and PHN may account for significant costs to the NHS. These initially represent the costs of dealing with the acute event and of the sequelae, particularly PHN. If the acute attack of shingles leads to hospital admission in a frail older person, there will be major costs, particularly since these patients may require longer hospital stays and rehabilitation. Ophthalmic shingles is fairly common and ophthalmology services are often involved. Patients may frequently present with the pain over two to three months after the rash and if persistent PHN is a problem, then there may be several therapeutic trials and further outpatient attendances. It is said the costs of managing herpes zoster and PHN in England and Wales amounts to over £47m, with acute zoster accounting for £32m and PHN for the remaining £15m.20
In a recent study the average overall costs of shingles over the first six months was calculated at £524 per patient. The medical costs associated with acute shingles was over £198 per patient, with 72% of the total cost accounted for by patients over 65 years, who cost on average £401 €” approximately five times as much as those under 65 years. The costs for older people were mainly due to NHS costs.21
Estimates of the burden of disease have been made, most notably in the SPS study, where a severity by duration measure of the total pain and discomfort associated with shingles was computed. It is clear early treatment is likely to impact upon subsequent events. The ideal option would be to prevent shingles where possible. It is interesting that the number of cases in the US has risen in more recent times.
It is not clear whether the same is the case in the UK. Given that shingles results from reactivation of VZV, it was suggested vaccination against VZV would decrease incidence and severity of shingles and PHN. The SPS study was designed to test this hypothesis. This was a large randomised double-blind placebo-controlled trial of a live attenuated varicella zoster vaccine involving 38,546 adults aged 60 years and over. It showed that zoster vaccine reduced the risk of developing shingles by 51%, reduced PHN incidence by 66.5% and also reduced the estimated burden of illness by 61%. These represent significant reductions in morbidity in an older population.
Conclusion
Shingles and PHN are debilitating conditions for older people. Shingles itself is associated with considerable complications and PHN is a distressing condition that can affect mood, daily function and quality of life for the patient as well as impact on any carers involved. PHN is difficult to manage and can be associated with signifi cant costs. The SPS study shows that zoster vaccine greatly reduces the morbidity and disease burden associated with shingles and PHN.
Key points
- Shingles is more common in older people due to an age-related decline in cell mediated immunity.
- Early presentation to primary care services is recommended so that antiviral agents can be started as soon as possible.
- Complications of shingles include ophthalmic and facial nerve involvement as well as the common problem of postherpetic neuralgia (PHN).
- PHN increases with age and often has profound effects on mood, sleep, general functioning and quality of life.
- A shingles prevention strategy should reduce complications and varicella zoster vaccination has been shown to signifi cantly reduce incidence of shingles, PHN and associated morbidity in older people.
Conflict of interest: Dr Passmore has served as consultee and/or received grants from Sanofi Pasteur MSD, Napp Pharmaceuticals and Grunenthal Ltd UK.
References
- Gnann JW, Whitley RJ. Herpes zoster. NEJM 2002;347:340-6
- Johnson RW, Whitton TL. Management of herpes zoster (shingles) and postherpetic neuralgia. Expert Opin Pharmacother 2004;5:551-9
- Johnson RW. Herpes zosterpredicting and minimizing the impact of post-herpetic neuralgia. J Antimicrob Chemother 2001;47:1-8
- Bowsher D. The lifetime occurrence of herpes zoster and prevalence of post-herpetic neuralgia: a retrospective study in an elderly population. Eur J Pain 1999;3:335-42
- Yih WK, Brooks DR, Lett SM et al. The incidence of varicella and herpoes zoster in Massachusetts as measured by the behavioural Risk factor Surveillance Ssytem (BRFSS) during a period of increasing varicella vaccine coverage, 1998-2003. BMC Public Health 2005;5:68:1-9
- Dworkin RH, Portenoy RK. Proposed classifi cation of herpes zoster pain. Lancet 1994;343:1648
- Haanpaa M, Dastidar P, Weinberg A et al. CSF and MRI fi ndings in patients with acute herpes zoster. Neurology 1998;51:1405-11
- Alper BS, Lewis PR. Treatment of postherpetic neuralgia. J Fam Pract 2002;51:121-8
- Watson CP Evans, RJ, Watt VR et al. Post-herpetic neuralgia: 208 cases. Pain 1998;35:289-97
- Helgasson S, Petursson G, Gudmundsson S et al. Prevalence of postherpetic neuralgia after a first episode of herpes zoster: prospective study with long term follow up. BMJ 2000;321:794-6
- Scott FT, Leedham-Greene ME, Winsome et al. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virology 2003;70: S24-S30
- Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Phys 2002;66:1723-30
- Hope Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract 1975;25:571-5
- Oxman MN, Levin MJ, Johnson GE et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. NEJM 2005;352:2271-84
- Coen PG, Scott F, LeedhamGreen et al. Predicting and preventing post-herpetic neuralgia: are current risk factors useful in clinical practice? Eur J Pain 2006;10:695-700
- Whitley RJ, Shukla S, Crooks RJ. The identifi cation of risk factors associated with persistent pain following herpes zoster. J Inf Dis 1998;178, Suppl 1:S71-S75
- Rowbotham MC, Petersen KL. Zoster associated pain and neural dysfunction. Pain 2001;93:1-5
- Dubinsky RM, Kabbani Z, ElCghami et al. Practice parameter: Treatment of postherpetic neuralgia: An evidence based report of the Quality Standards Subcommittee of the American Academy of neurology. Neurology 2004;63:959-65
- Finnerup NB, Otto M, McQuay HJ et al. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118:289-305
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential costeffectiveness of vaccination in England and Wales. Vaccine 2000;19:3076-90
- Scott F, Johnson RW, LeedhamGreen M et al. The burden of herpes zoster: a prospective population based study. Vaccine 2006;24:1308-14



Comments are closed.